Welcome to LifeCourse!
The Melbourne Children’s LifeCourse Initiative brings together an impressive array of longitudinal cohort studies from the Melbourne Children’s Campus.
This vital platform equips researchers with the tools, collaborations and access to produce more meaningful and robust research.
Explore the outstanding cohorts and data available through the LifeCourse platform to uncover the potential value-add for your research.
About LifeCourse
LifeCourse supports over 40 studies including 25 core longitudinal cohorts spanning conception to adulthood (including two trans-generational studies) and involving over 80,000 participants, with many enriched through collection of biosamples, imaging, and linkage to administrative data. By bringing these data resources together we continue to build our understanding of the burden, causes and consequences of health and disease from early life.
LifeCourse is jointly supported by The Melbourne Children’s Campus partners: The Royal Children’s Hospital, Murdoch Children’s Research Institute (MCRI) and The University of Melbourne (Department of Paediatrics), established with funding from the Royal Children’s Hospital Foundation.
Our Research & Impact
Health and wellbeing are complex, and vary across the lifespan, with many health challenges starting from childhood. Therefore, LifeCourse cohorts are well positioned to address the development of health outcomes, as they typically:
- Start from early life
- Follow children’s health and development over time
- Capture complex influences across children’s environments and experiences
Focus research areas
The LifeCourse platform provides researchers with particularly rich data in the three key health areas of mental health, cardiometabolic health and immune health. Additionally, since 2020 the implications of COVID-19 has emerged as a key area of focus and collaboration for the LifeCourse cohorts:
LifeCourse Publications
LifeCourse researchers publish many papers detailing new discoveries every year, capitalising on the strengths of our rich cohorts to address complex health issues.
In fact, over 750 publications have been generated from the data in our LifeCourse cohorts. These have been published in some of the world’s leading peer-reviewed journals, and cited nearly 33,000 times in the literature.
Last updated on 12/08/2025.
Most-published in journals by LifeCourse cohorts, by impact factor, as at 12/08/2025.
Below are examples of our recent publications, which illustrate the potential of the LifeCourse platform to enable research that wouldn’t otherwise be possible.
LifeCourse Data Resource Profile

Learn about the features and contributions of the longitudinal cohorts which represent the LifeCourse Initiative.
Approaches to analysing data across cohorts

How can researchers draw multiple cohorts together to better address their research questions?
Understanding causation in
multi-cohort studies

The CEBU team consider causal inference and the role that the target trial can play in the multi-cohort setting.
Impacts of COVID-19 for young people

LifeCourse cohorts provide considerations for policy development to address the impacts of COVID-19.
Positive mental health in adolescence

LifeCourse researchers dive into the emerging field of positive epidemiology.
Impact of our research
Our ambitious goal is to enable impactful research that benefits health across the life span. LifeCourse cohorts influence health through a range of pathways relating to research, policy, service system, and societal impacts.

Reproduced from O'Connor et al. (2025). Key domains of research impact with illustrative (but not exhaustive) examples particularly relevant to cohort studies, adapted from Kuruvilla et al. (2006).
Our cohorts are generating attention across all of these domains. For example, LifeCourse publications are helping to raise awareness and attention to health over the life span through more than 2,000 news stories and nearly 10,000 social media mentions.
Mentions of LifeCourse papers by forum, as at 12/08/2025.
Policy influence
A particular area of focus for our LifeCourse cohorts is influencing health, social, and educational policy. Our cohort data have the richness and rigour to inform policy and decision-making in many areas.
Our cohort teams engage in a wide range of activities to ensure that decision makers have the evidence they need at hand. Research briefs and parliamentary submissions are common examples of such activities.
| Research briefs | Parliamentary submissions |
|---|---|
|
Non-technical summaries of research findings. We make our Briefs publicly available and share these with policy and decision-makers to communicate the evidence-base in support of a particular issue. |
Parliamentary submissions are reports directly submitted for consideration to Parliament to address a specific policy that is up for debate. |
| The impact of COVID-19 on children's wellbeing | |
|
Research brief: Mental health impacts of the COVID-19 pandemic for children and young people (December, 2022) This research brief provides considerations for policy development to address the psychosocial impacts of COVID-19. Data from LifeCourse cohorts demonstrated the need to leverage integrated services and systems to address mental health problems, ensure continuity of mental health care, address socioeconomic disadvantage, and continue to collect longitudinal data to understand recovery. |
Figure 1: Parliamentary inquiry into state education system in Victoria (November, 2023) This parliamentary submission provided evidence on children’s learning and wellbeing. Evidence from LifeCourse cohorts was used to demonstrate the how children and their families can be best supported to enhance resilience. Recommendations were put forward to improve the services and organisations that children and young people interact with. |
Reflecting the work our cohorts do to engage with policy development, we see evidence of our cohorts’ research being cited in policy documents internationally. LifeCourse data has proven to be particularly impactful in policies for drug and substance use, child and adolescent development, and mental health and wellbeing.

Number of policy documents citing LifeCourse papers by topic, as at 18/1/2024.
Notably, leading authorities on global health like the World Health Organisation are looking to LifeCourse to inform their evidence base. This is just one example of the exceptionally generative and far-reaching utility of LifeCourse cohorts.
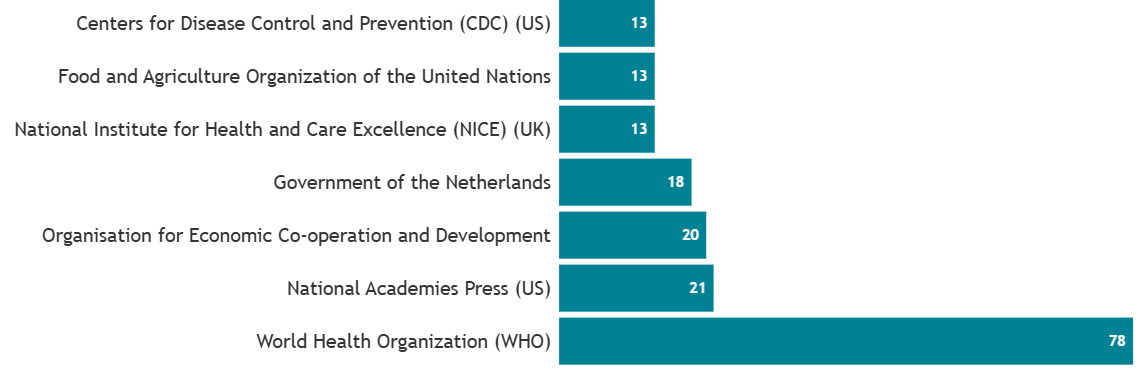
Policy organisations citing LifeCourse papers, top 5 as at 12/08/2025.
Interested in thinking through how your cohort can maximise their real-world impact? Visit the Melbourne Children’s Impact Hub to begin your journey. Researchers from the Melbourne Children’s Campus can also contact the Impact team and join the Knowledge Translation and Impact Network.
Community of Practice
At LifeCourse, we're dedicated to advancing life span health through collaborations that maximise the benefits of existing data. We strive to grow and foster a thriving community of cohort researchers to share knowledge and best practice approaches. Explore how you can get involved below.
LifeCourse Forums
LifeCourse run forum sessions connecting longitudinal researchers and exploring topics relevant to cohorts from across the Melbourne Children's Campus and beyond. Join the LifeCourse mailing list to attend! All are welcome.
A curated back catalogue of recordings of previous sessions (when available) is accessible on the LifeCourse Forums Hub.
LifeCourse Cohort Forum
The LifeCourse Cohort Forum features expert presenters from the Melbourne Children’s Campus and beyond, tackling cross-cutting issues relating to longitudinal research, analysis, and cohort management. LifeCourse aims to facilitate the sharing of knowledge, experiences and common challenges among researchers through our vibrant community of practice. All researchers and research support staff are welcome to attend. We extend a sincere thanks to our current and previous contributors for their generous support and contribution into this series, including presenters from the Clinical Epidemiology and Biostatistics Unit (CEBU) for their invaluable input on statistical topics.
| Date | Presenter/s | Topic |
|---|---|---|
| 12/02/2025 | Ben Edwards, Kate Doery | Increasing response rates in youth longitudinal surveys: GENERATION’s Wave 3 |
| 12/03/2025 | Nigel Curtis | Surviving the Academic Hunger Games: Writing in a Publish-or-Perish World |
| 24/03/2025 | Josie Dickerson |
Born in Bradford: A family of cohort studies designed to change a city |
| 9/04/2025 | Fran Azpitarte |
Evaluating the impact of policy reform on children with special educational needs in England using data linkage and statistical analysis |
| 14/05/2025 | Nitya Philipson, Cathy Quinlan |
From Bench to Bedside to Blackboard - AI’s Expanding Role in clinical care, teaching and research |
| 11/06/2025 | Kate Wyatt |
Involvement of children and young people in longitudinal health research – Learnings from cohort consultation for GenV |
| 1/07/2025 | Melissa Wake, Will Siero, Valerie Sung | Spotlight on GenV - where have we been, where are we going and how can we collaborate? |
| 9/07/2025 | Rushani Wijesuriya | Handling missing data in longitudinal studies using multiple imputation |
| 20/08/2025 | Suzanne Mavoa | Introductory workshop: Geospatial data and analyses in health research |
| 29/10/2025 | Meredith O'Connor, Elodie O'Connor | Playing the long[itudinal] game: Research impact in cohort studies |
| 25/11/2025 | Melissa Wake, Will Siero | Spotlight on GenV - the Early School Wave in Action |
| 14/01/2026 | Evangeline Tabor |
Impact for longitudinal studies and research: a UK perspective on theorising and measuring the influence of cohort studies |
LifeCourse Cohort Forum sessions in 2025-2026.
Data Linkage Interest Group
Aims to connect researchers analysing linked data or tackling various aspects of the data linkage process. Presentations help to showcase and troubleshoot a wide array of linkage issues in research.
| Date | Presenter/s | Topic |
|---|---|---|
| 30/04/2025 | Anna Price | Using linked data at school transition to evaluate the right@home trial |
| 08/09/2025 | Sharmani Barnard | Q&A with Sharmani Barnard |
| 30/09/2025 | Andrew Boyd | UK Longitudinal Linkage Collaboration: the national Trusted Research Environment for the longitudinal research community |
LifeCourse Data Linkage Interest Group sessions in 2025.
Have a topic to suggest, or want to present at one of our forums?
Leave a note in our Suggestions Box or contact the LifeCourse team!
Cohort Management
Managing each aspect of your cohort study is no small task. Whether it’s the design of your research, translation of your outcomes, legal agreements or data management, there are resources available to assist you on your way.
The LifeCourse team works to develop resources that support cohort researchers in particular, who are faced with unique and complex challenges due to the longitudinal design of their work. Our aim is to help cohort researchers maximise the use of their rich and robust cohort data, and increase efficiencies within cohort teams.
See our LifeCourse-developed resources below, publicly available to all researchers.
MCRI researchers can also access the wealth of expertise and resources available across campus. An MCRI login may be required to access some of these internal resources.
LifeCourse-developed resources
Ethics and governance
New and existing cohort studies are required to maintain the highest standards of ethical conduct. Ethics approval for each of the LifeCourse cohorts is granted through the Royal Children's Hospital Human Research Ethics Committee (RCH HREC).
The Royal Children’s Hospital Research Ethics and Governance (REG) Office have templates and guides to support your next ethics application or amendment. This includes guidance on informed consent and how to write in plain language.
The Clinical Research Development Office (CRDO) also provides education and training for researchers to develop their skills, manage their research and adhere to regulatory frameworks.
Common ethical issues for longitudinal cohort studies include:
- Negotiating ongoing consent models
- Requirements for secondary data analysis
- Participation engagement, retention and tracing
In longitudinal study designs, negotiating ongoing consent models is crucial to keeping participants engaged over time.
Particular challenges include navigating the transition from parent consent to young adult consent, and updating participants about changes to how their data will be collected, stored, and utilised. The Research Ethics and Governance (REG) office can assist you with those challenges.
Planning to collect additional data from your cohort, or access data from another cohort for analysis? If your answer is yes, you need ethics approval.
If requesting data for secondary data analysis, data users from outside the cohort’s study team will need to consider whether their intentions for the data extend beyond the study’s original aims. If so, it is the data users' responsibility to explore the submission of a new ethics application covering secondary analysis of the data. Some cohorts may require a copy of the approval letter and protocol before transferring the data.
For an example of an ethics protocol for secondary data analysis, view the COVID Wellbeing ethics protocol here, developed by LifeCourse data users.
Participant engagement, retention and tracing is also a key issue in longitudinal cohort study design. Cohorts often implement a wide range of strategies to engage and trace their participants. This may including tailored communications, considered timing of contact, accessing public databases or use of social media. These strategies may need to be approved by REG office before implementing.
Additionally, LifeCourse hosts forum sessions on topics pertaining to ethics & governance in cohort studies. Sign up to view recordings of our previous presentations on our Forums Hub.
Funding
The MCRI Grants Office can assist in finding funding opportunities, provide advice on applications, access templates, and ensure all administrative and legal requirements are followed.
When applying for a grant, it is important to contact the Grants team as early as possible to discuss your application and benefit from the support they can offer.
Legal
Whether you are in need of a material transfer agreement, confidentiality agreement, research collaborative agreement, services agreement, contractor agreement or bespoke agreement, the Legal team have got you covered.
MCRI researchers can visit the Legal page to find helpful templates for a range of agreements or email the Legal team to explore issues specific to your study.
Research design
Appropriate and considered design of your research is important to maximise the utility and translation of your data. Survey design is a crucial element, particularly for longitudinal studies, as researchers have the opportunity to tell a story over time through their cohort data. Researchers need to balance consistency through the surveys for comparability, while making sure each survey is age-appropriate and collects relevant data for their cohort at that time point.
The LifeCourse Measurement Library is a great tool to understand the measures already collected within the LifeCourse cohorts. 24 priority measures have also been identified, with key information made available (such as cost per use, time to complete, reference frame).
MCRI researchers can explore whether a site-wide licence has been obtained for a measure they are interested in using. If not, there may be scope to acquire one. Contact the Data Office to consider your options.
The LifeCourse team have also collated a Standard Demographics Module, input into REDCap by the Data Office, and released with an accompanying data dictionary. This means that these survey items can be easily input into your REDCap project and integrated into your study documentation.
Research translation
Longitudinal studies have the potential for significant research impact. Unlike other study designs, they can track health and wellbeing trajectories over time.
It is crucial to consider your plan for impact from the outset of your longitudinal study. Learn more about translation and impact via the campus Research Hub. These resources will help you map out a feasible and comprehensive plan for research impact. You may also consider joining the Melbourne Children’s Knowledge Translation and Impact network, which is a campus community group that shares tools, tips, and examples of good practice in this area.
Looking to develop a product, learning tool or an app as part of your impact plan? The Innovation team can help you develop and translate your research ideas into real-world applications. Learn more about concepts like intellectual property, commercialisation, and how to engage with investment and industry partners.
LifeCourse cohorts are influencing health through a range of pathways relating to research, policy, service system, and societal impacts. See Our Research & Impact for examples.
Looking for novel and accessible ways to communicate your research findings with non-scientific communities? Access Plain Language Summary tools here, described by Lottie Gasparini in her presentation to the LifeCourse Cohort Researchers Forum.
Data management
Whether you are setting up a new cohort or updating existing practices, developing good data management practices will save you time and effort in the long run. To ensure you are adhering to best practice standards, there is no shortage of resources and services on campus to help you along the way.
DataConnect is a great starting point to connect you with tools, resources and knowledge for a more informed data journey. MCRI researchers are required to populate a Data Management Plan (DMP) which ensures alignment with MCRI's Data Governance Framework (DASUD).
LifeCourse have also developed key resources to help cohort researchers plan, design and share their research:
- Data Dictionary Resource to help cohorts design best-practice data dictionaries
- Data Sharing Planning Resource to help plan for future data sharing
Data analysis
Longitudinal data analysis can be uniquely challenging. The Melbourne Children's Campus is home to the Clinical Epidemiology and Biostatistics Unit (CEBU), an internationally renowned methodological hub for expertise in data management and analysis, particularly biostatistics and epidemiological methods.
Getting high-level statistical input on your project from its inception to publication is critical in cohort research. This includes:
- Clarification and refinement of the research question
- Development of an analysis plan, including epidemiological design principles and selection of the most appropriate methods, access CEBU's statistical analysis plan for observation studies
- Reporting and interpretation of analyses
CEBU also run short courses and training programs to upskill researchers in analytic methods and the use of statistical analysis software.
Additionally, LifeCourse have developed a resource to assist cohorts in making their code open and accessible.
To request access to data from any of our LifeCourse cohorts, visit the Data Access page to submit an initial enquiry and get the ball rolling!
Harmonisation
Data harmonisation refers to the process of aligning comparable variables across studies. This allows researchers to repeat analyses across cohorts, or even pool these aligned data into one larger dataset for analysis.
With bigger datasets and sample sizes, there is greater potential for impact, leading to more timely outcomes, and a reduced need for new, costly data collections!
However, harmonisation can also be scientifically complex and raises some interesting methodological questions. Researchers at the Melbourne Children’s Campus can also seek expert advice from the CEBU team to work through statistical challenges. In fact, CEBU are global thought leaders in how we can rigorously think through data harmonisation decisions.
How does data harmonisation happen?
Researchers are combining data from across studies to answer some of the biggest and most complex questions facing child and adolescent health, that could not be answered by a single cohort alone.
Existing data can be together from different cohorts to retrospectively align different measures or survey items that capture the same meaning.
Or, for more seamless alignment, cohort teams can plan for harmonisation before collecting data by using the same measures when aiming to capture the same information.
The latter - deliberately harmonising data across multiple cohorts in a planned way is a particularly timely, cost-efficient and ethical way to maximise the use of pre-existing data.
How does LifeCourse support harmonisation?
LifeCourse aims to make data harmonisation easier for those looking to undertake it. See which measures have been used by which cohorts, and when, by ‘Exploring our cohorts’.
Find a comprehensive and searchable list of all the measures used within the LifeCourse cohorts in the Measurement Library. Here you will also be able to see which measures are the most commonly used within each domain. Read more about how we identified these 24 common measures in our accompanying report. Also find useful information for these common measures, such as cost per use, time to complete, or reference frame. This information will provide a starting point in understanding whether this measure could be appropriate for use in your own research.
The LifeCourse team have also collated a standard demographics item set, programmed into REDCap by the Data Office, which builds on the methodical work from other large cohort studies, like GenV and the Longitudinal Study of Australian Children. This resource also comes with a companion data dictionary and is already programmed into REDCap for easy implementation.
Additionally, the LifeCourse team hosted researchers from the UK to introduce Harmony, a free-to-use tool which utilises natural language processing to match items across different measures and questionnaires. This allows data users to discover the potential to harmonise across studies in an efficient way. Revisit their recent presentation to the LifeCourse Cohort Researchers Forum.
Supporting Resources
Open Science
Open Science describes an ongoing journey towards increasing the transparency of research processes to advance scientific discovery. LifeCourse supports cohorts and researchers to move in this direction and align with the FAIR principles, while ensuring that best practice ethical and data security standards are maintained at all times.
Open Science includes a number of key principles which are increasingly being adopted throughout LifeCourse working practices. Keep reading to learn more.

Data Dictionary Resource for LifeCourse cohorts
Open materials is about making components of research methodology publicly available, including what data was collected and how it was collected. This makes study findings more transparent and reproducible.
Our Data Dictionary Resource helps custodians to set up their data dictionaries in adherence with best-practice standards, minimising the duplication of data management work in the future.
Open Code: Promoting Efficiency and Reproducibility of LifeCourse Analyses
Open code involves sharing the code used to analyse data, which promotes efficiency, ensures knowledge isn’t lost when staff move on, and supports transparency and reliability. Sharing analytic code can promote visibility, citations, and allow researchers to publish in those top journals for which code sharing is a requirement.
Our Open Code resource describes the strengths and key considerations of code sharing and how it contributes to an environment of open science and collaborative research.

Open conversations about Open Data: Planning for data sharing in LifeCourse cohorts
Balancing the value of transparency with the need to protect participant data is critical to sharing data responsibly.
LifeCourse has a clear and transparent procedure for researchers to apply for access to the data within our cohorts, that also ensures all safeguards for participants are maintained.
Our guide on Planning for data sharing is helping data custodians to streamline and future-proof their data sharing approaches.
How does the MCRI support Open Science?
LifeCourse’s efforts are well aligned to those of our host organisation, the Murdoch Children’s Research Institute. The MCRI is committed to promoting Open Science principles, and has policies and procedures to facilitate compliance with the Open Access requirements set by MCRI’s research funders like the NHMRC and ARC.
For more information, see the DataConnect Open Access page. An MCRI login may be required to access some of these internal resources.
Supporting Resources
Icons used above for Open Science, Code and Data were developed by the Center for Open Science.
Meet the Team
A small and efficient team work to maintain and optimise the LifeCourse infrastructure, as well as undertake research that demonstrates the power of the platform to generate new insights into health pathways over the life span.

Dr Meredith O'Connor - Head of LifeCourse Cohort Infrastructure & Senior Research Fellow
Meredith directs the LifeCourse data infrastructure, developing systems that enable reuse of these valuable longitudinal data assets to facilitate and foster real world impact. Her research program exemplifies the capacity of LifeCourse cohorts to advance understanding of health over the life span, with a particular focus on how cohorts can be analysed in tandem to gain new insights. In addition, she contributes to the strategy and design of prominent longitudinal studies in Australia, including serving as a Scientific Advisor for the Longitudinal Study of Australian Children since 2018.

Tehani Paiva - LifeCourse Initiative Coordinator
Tehani joined the LifeCourse team in January 2019. She currently manages key LifeCourse projects that develop data infrastructure and resources supporting LifeCourse researchers. She has specialised knowledge in the measures and instruments used by cohorts, and manages the cohort metadata catalogue where researchers can browse this information. She also works with cohort custodians to foster the sharing of knowledge and approaches across teams, and assists in the curation of the LifeCourse forums program.

Ella Woodbridge - Project Assistant
Ella joined the LifeCourse team as a Project Assistant in 2024. In this role, she liaises with cohort custodians to update their metadata, manages and promotes LifeCourse forums, and maintains LifeCourse website resources. She has a background in education and disability support. She is undertaking a Master of Public Health and is interested in health intelligence and reproductive health.
Scientific Leads and Champions
The LifeCourse team is supported by leading researchers who provide expertise across diverse research disciplines. They play a pivotal role in supporting the LifeCourse initiative by identifying innovative research directions, particularly participation in international cross-cohort consortia; advocating for the platform; ensuring alignment with other Campus efforts; and fostering relationships with key stakeholders.

Professor Craig Olsson (Founding Investigator) - Mental health
Craig led the early development of LifeCourse between 2014-2018 as founding investigator of the LifeCourse platform. He holds an NHMRC Investigator Grant and leads The Australian Temperament Project Generation 3 Study (est. 1983), one of Australia’s longest running studies examining the developmental origins of mental health and disorder. He also leads the Intergenerational Cohort Consortium of multigenerational cohort studies, and is National Convenor of the Australian Research Alliance for Children and Youth (ARACY) Longitudinal Studies Network.

Professor David Burgner (Founding Investigator) - Infection, inflammation and chronic disease
Dave is a paediatric infectious diseases clinician scientist. He holds an NHMRC Investigator Grant and is one of the lead researchers on the Barwon Infant Study, Child Health Checkpoint and a number of COVID-19 related projects. He also co-leads a number of national and international cross-cohort studies. His research focus is on understanding early life determinants of infection and inflammation, and their role in the development of conditions like heart disease and obesity.

A/Professor Margarita Moreno-Betancur - Research methodology
Margarita is co-Director of the Clinical Epidemiology and Biostatistics Unit (CEBU). She leads an integrated program of methodological and collaborative research, currently supported by an NHMRC Investigator Grant (2022-26). With the goal of bringing methodological excellence to observational research on campus, she has developed a hub of innovation and expertise in statistical areas that are key for these studies, such as causal inference.

Professor Andrew Sinclair - Data infrastructure
Andrew is Deputy Director of the Murdoch Children's Research Institute (MCRI) and a Professor in the Department of Paediatrics at The University of Melbourne. In these roles and as Executive Director of the Victorian Clinical Genetics Service at the Royal Children's Hospital, Melbourne, he is using genomic information to improve healthcare for children and their families. He also leads MCRI’s efforts to support best-practice data management and governance approaches.
Other critical contributors
LifeCourse is a collaboration between the Murdoch Children’s Research Institute (MCRI), the University of Melbourne, and the Royal Children's Hospital, located at MCRI. We acknowledge all collaborators who have contributed to LifeCourse, especially cohort data custodians and their participants, and our LifeCourse Initiative funders, the Royal Children's Hospital Foundation and MCRI. LifeCourse is a collaborative effort, and we would like to thank everyone for their ongoing engagement and support.
Special thanks to these previous and ongoing contributors to LifeCourse:
Executive champions
Sharon Goldfeld
Andrew Sinclair
Past leadership
Katie Allen
Leanne Mills
Melissa Wake
Advisory group members
Andrew Grimes
Anne-Louise Ponsonby
Ben Ong
Jatender Mohal
Jennifer Piscionere
John Carlin
Justine Ellis
Kate Lucas
Luke Stevens
Michael Poidinger
Nitya Phillipson
Revital Rosenberg
Richard Saffery
Shyamali Dharmage
Steven McConchie
Research contributors
Harindra Jayasekara
Katherine Lange
Marnie Downes
Sarah Arnup
Project contributors
Alana Deery
Angela Pezic
Anna Booth
Anna Duncan
Emma Jarosz
Gabriella Tikellis
Hannah Mcglashan
Joanne Ryan
Keri Little
Kerry Haynes
Shirani Sivarajah
Will Siero
Web development
Adam Leadoux
Dhanuka Kaluarachchi
Hilianty Tardjono
Lucy Anderson
Vijay Mudapilaval Rajendranath
Postdoctoral Fellows and PhD students
In 2019, with the support of the Royal Children’s Hospital Foundation, LifeCourse awarded nine part-time postdoctoral fellowships and three PhD scholarships to conduct a diverse range of research projects using LifeCourse data. The LifeCourse PhD and Postdoctoral Support Program is now completed, having delivered increased Campus capability and connections in longitudinal and life course research. Many PhD students and Postdoctoral Fellows continue to use LifeCourse data to shape their research, supported by a range of other funding streams.
LifeCourse Postdoctoral fellows (2019-2021)
| Fallon Cook | Infant regulation and child mental health |
| Izabela Fedyszyn | The natural history of self-harm from childhood to adolescence and its predictors |
| Jessica Kerr | The interaction of obesogenic genes and obesogenic neighbourhood environments in predicting childhood obesity and metabolic syndrome |
| Jing Wang | To what extent does genetic and environmental risk influence language outcomes at different levels of hearing? |
| Katherine Lange | "Happy and Healthy” – molecular profiles of mental wellbeing in childhood and early adulthood |
| Laura Conway | Early life adversity and children’s language and learning |
| Melanie Neeland | Early life immune biomarkers of childhood allergy |
| Mihiri Silva | Understanding the origins and impact of dental health: a life course approach |
LifeCourse PhD students (2019-2022)
| Cindy Pham | Prenatal oxidant exposures, oxidative stress and the integration of mitochondriomics in childhood neurodevelopment |
| Heidi Renner | Identifying and reducing inequities in educational pathways through socially inclusive community practices |
| Nur Sabrina Idrose | The effects of pollen exposure on lung function and airway inflammation in children and adults, and potential modifiers of these associations |



















































