Welcome to LifeCourse!
The Melbourne Children’s LifeCourse Initiative brings together an impressive array of longitudinal cohort studies from the Melbourne Children’s Campus.
This vital platform equips researchers with the tools, collaborations and access to produce more meaningful and robust research.
Explore the outstanding cohorts and data available through the LifeCourse platform to uncover the potential value-add for your research.
About LifeCourse
The Melbourne Children’s LifeCourse Initiative
The Melbourne Children’s Campus is a global leader for longitudinal and life course data, creating an unparalleled resource for informing policy and service development.
LifeCourse supports over 40 studies including 23 core longitudinal cohorts spanning conception to adulthood (including two trans-generational studies) and involving over 40,000 participants, with many enriched through collection of biosamples, imaging, and linkage to administrative data.
These studies have already shaped policy and clinical care nationally and internationally. By bringing these data resources together we continue to build our understanding of the burden, causes and consequences of health and disease from early life.
LifeCourse is jointly supported by The Melbourne Children’s Campus partners: The Royal Children’s Hospital, Murdoch Children’s Research Institute (MCRI) and The University of Melbourne (Department of Paediatrics), established with funding from the Royal Children’s Hospital Foundation.
Forums and Training
Did you know?
LifeCourse hosts a range of forums that provide space for synergies to thrive across the research community.
LifeCourse is supporting the development of all longitudinal researchers.
LifeCourse run several forums connecting longitudinal researchers with each other and experts in topics relevant to cohorts from across the Campus and beyond.
How do I get involved?
Anyone is welcome to attend the LifeCourse forums. Want to be reminded of upcoming sessions via our mailing list? Sign up here.
Visit the Data Management and Analysis section, for more information about training programs offered through CEBU.
LifeCourse Forums
The LifeCourse Forums Hub provides a list of previous sessions and hosts a back catalogue of recordings of previous sessions (when available).
Contact the LifeCourse team if you want to suggest a topic of interest or would like to present at one of our forums.
Sign up here to join the mailing list and attend our forum sessions.
LifeCourse Cohort Researchers Forum
Curated in partnership with CEBU, this series draws in expertise from presenters across the campus and beyond, focusing on cross-cutting issues relating to cohort research and analysis that transcend specific content areas, such as modern analytic methods relevant to cohort research. This program has been primarily designed to support researchers working with cohort studies and data.
| Date | Presenter | Title |
|---|---|---|
| 12/02/2024 | Nitya Phillipson, Nauman Maqbool | Embracing Open Science - starting with Open Access! |
| 05/03/2024 | Bettina Moltrecht, Eoin McElroy | Harmony - A global platform for contextual harmonization, translation and cooperation in mental health research |
| 03/04/2024 | Stephanie Cahill | Introduction to Growing Up in Digital Europe (GUIDE): Measuring Wellbeing from Birth to 24 years |
| 08/04/2024 | Revi Rosenberg | Charting Progress: MCRI's Data Evolution Over the Past Two Years and Plans for the Future |
| 15/04/2024 | Ken Knight, Nyanhial Yang, Leanne Constable |
Involving consumers, community & children in research - Emerging principles & good practice approaches |
LifeCourse Coordinators Forum
Provides an opportunity for cohort coordinators and managers to meet, connect, and share knowledge about operational and project issues relevant to cohort studies. Sessions are generally more informal and discussion based. This forum has been primarily designed to support those working in operational and project roles on cohort studies.
| Date | Presenter | Title |
|---|---|---|
| 28/02/2024 | Meredith O'Connor |
Planning for data sharing - a pragmatic guide for cohorts |
| 30/03/2024 | Peter Xiberras |
New Systems & Software Approvals |
| 15/05/2024 | Steven McConchie |
Data Management Planning at MCRI |
LifeCourse Data Linkage Interest Group
Aims to connect researchers working on, or planning various aspects of data linkage. Feature presentations are also scheduled to showcase and troubleshoot a wide array of linkage issues in research.
| Date | Presenter | Title |
|---|---|---|
| 26/02/2024 | Ashleigh Shipton |
Navigating the Data Maze: Lessons in obtaining and linking life course data within CVDL and MCH |
| 25/03/2024 | Bridget Healey, Luke Collier |
Leveraging linked data to promote learning and development through Early Childhood Education and Care (ECEC) pathways |
LifeCourse Postdoctoral Fellows and PhD students
In 2019, the LifeCourse PhD and Postdoctoral Support Program was launched to build campus capacity in skills and methodologies relevant to cohort data.
With the support of the Royal Children’s Hospital Foundation, LifeCourse awarded nine part-time postdoctoral fellowships and three PhD scholarship to conduct a diverse range of research projects using LifeCourse data.
The LifeCourse PhD and Postdoctoral Support Program is now completed having delivered increased Campus capability and connections in longitudinal and life course research. Find out more about the impactful research undertaken by our Fellows and students below.
Many PhD students and Postdoctoral Fellows continue to use LifeCourse data to shape their research, supported by a range of other funding streams. LifeCourse continues to support these researchers in the development of their skills in working with longitudinal cohorts via our forums and knowledge hubs.
Postdoctoral fellows
| Fallon Cook | Infant regulation and child mental health |
| Izabela Fedyszyn | The natural history of self-harm from childhood to adolescence and its predictors |
| Jessica Kerr | The interaction of obesogenic genes and obesogenic neighbourhood environments in predicting childhood obesity and metabolic syndrome |
| Jing Wang | To what extent does genetic and environmental risk influence language outcomes at different levels of hearing? |
| Katherine Lange | "Happy and Healthy” – molecular profiles of mental wellbeing in childhood and early adulthood |
| Laura Conway | Early life adversity and children’s language and learning |
| Melanie Neeland | Early life immune biomarkers of childhood allergy |
| Mihiri Silva | Understanding the origins and impact of dental health: a life course approach |
PhD students
| Cindy Pham | Prenatal oxidant exposures, oxidative stress and the integration of mitochondriomics in childhood neurodevelopment |
| Heidi Renner | Identifying and reducing inequities in educational pathways through socially inclusive community practices |
| Nur Sabrina Idrose | The effects of pollen exposure on lung function and airway inflammation in children and adults, and potential modifiers of these associations |
Our Research
Did you know?
LifeCourse cohorts make meaningful impact by informing policy and practice across Australia.
LifeCourse encourages and facilitates collaborations across cohorts and disciplinary boundaries.
Featured LifeCourse Publications
Read the LifeCourse Data Resource Profile

Learn about the features and contributions of the longitudinal cohorts which represent the LifeCourse Initiative.
Approaches for analysing data across cohorts

How can researchers draw multiple cohorts together to better address their research questions?
Using the target trial approach in
multi-cohort studies

The CEBU team extend the target trial approach to the multi-cohort setting.
Psychosocial Impacts of COVID-19

Our research brief provides considerations for policy development to address the psychosocial impacts of COVID-19.
Focus research areas
The LifeCourse platform boasts exceptional data and collaborations across teams and cohorts in three key areas, which are overlayed with an equity and translation lens:
 Mental Health
Mental Health
Preventing mental health issues is integral. The breadth of LifeCourse studies at MCRI provides the opportunity to target the pathways that can lead to poor mental health across different ages, backgrounds and situations.
 Cardiometabolic Health
Cardiometabolic Health
The pathways to developing cardiometabolic health problems begin during pregnancy and early childhood, with childhood obesity being one of the biggest predictors. Supporting healthy habits and lifestyle from a young age, and maintaining this through adolescence provides the foundation for heart health right through the life course.
 Immune Function
Immune Function
With more and more Australian children being diagnosed with conditions stemming from alterations in immune function – like allergies, food intolerances and asthma – than ever before, MCRI researchers are on the hunt to uncover the reasons behind this, and to find novel treatments or cures.
COVID-19 Research Response
COVID-19 research emerged as a key area of focus and collaboration for LifeCourse, the Melbourne Children’s Campus and our cohorts in 2020. As part of the Melbourne Children’s response, LifeCourse facilitated the collection of additional COVID-related data from LifeCourse cohorts. These data provide a unique opportunity to understand how pre-pandemic experiences shape risk and resilience for children and young people during the crisis, and in the long-term aftermath.
Key publications using LifeCourse data
We publish many papers every year, drawing on data from our LifeCourse cohorts and covering numerous topics relating to health and development.
Here you will find just a selection of our recent publications, which illustrate the potential of the LifeCourse platform to enable:
- Research using more than one cohort (e.g. harmonising and pooling data from multiple cohorts, or replicating analyses in multiple cohorts)
- Research crossing traditional disciplinary boundaries and spanning multiple focus areas
Understanding the LifeCourse platform
Data Resource Profile
Read more about the features and contributions of the longitudinal cohorts which represent the LifeCourse Initiative in our Data Resource Profile. In this overview of the initiative, we describe how we are striving to improve child health and wellbeing by strengthening cohort infrastructure.
Influences on health and development through the life course
How has COVID-19 impacted children’s mental health?
During the COVID-19 pandemic, the LifeCourse cohorts worked collaboratively to understand impacts of widespread disruptions for the mental health and wellbeing of children, young people, and their families. Our research brief summarises research findings to date, and what these insights suggest for policy and service provision as we navigate the post-COVID recovery period.
How do children’s experiences of adversity ‘get under the skin’?
Researchers used data from the Barwon Infant Study and the Longitudinal Study of Australian Children Child Health CheckPoint to understand how children’s experiences of adversity ‘get under the skin’ to impact health outcomes. By using two cohorts that capture different age ranges, researchers were able to provide evidence of a link between adversity and chronic inflammation in both mid childhood and late childhood. Read more about this study here and relevance for the post-COVID-19 recovery here.
The transmission of mental health problems across generations
Spry et al. (2019) pooled data from two rare 3-Generation preconception cohorts: the Australian Temperament Project, Generation 3 and the Victorian Intergenerational Health Cohort Study. This intergenerational data allowed researchers to explore whether a history of depression before conception is linked to vulnerability to mental health problems in infants. Pooling data from across two cohorts gave researchers a unique opportunity to explore intergenerational outcomes in a sufficiently large sample. Read more here.
Development of mental health strengths
While preventing and intervening on mental disorder is essential, we also need to promote positive aspects of children’s mental health. Data from the Longitudinal Study of Australian Children and Millennium Cohort Study in the UK revealed that, in general, mental health competence steadily increases between childhood and adolescence. However, some children experience other pathways, such as declining competence during this period, and this was more common for boys and children from socially disadvantaged backgrounds. Read more here.
What are the consequences of early disadvantage for children’s development?
Study researchers found consistent evidence that socioeconomic disadvantage experienced in infancy can have a lasting impact on cognitive development, even if circumstances later change. Findings were consistent across the Australian Temperament Project and Longitudinal Study of Australian Children, despite these cohorts being recruited two decades apart. Read more here.
Approaches to working across cohorts
A review of opportunities for analysing data across cohorts
The LifeCourse team have reviewed the many different ways that researchers can work across cohorts to tackle research questions relevant to life course health and development. Drawing multiple cohorts together for analysis can further enhance the contribution of longitudinal cohorts. However, key challenges also need to be recognised and carefully managed.
Using the target trial approach for causal inference in multi-cohort studies
LifeCourse methodology lead and A/Prof Margarita Moreno-Betancur has previously discussed the possibilities for informing potential interventions by using the “target trial” approach in longitudinal cohort studies. The CEBU team, in collaboration with LifeCourse, now extend this approach to the multi-cohort setting. This provides a framework to address challenges specific to the multi-cohort setting such as clear identification of potential sources of bias, both within and between cohorts, which then guides analysis planning and informs the interpretation of findings.
Approaches to comparing disease prevalence and risk factors across cohorts
Comparing the frequency of a disease between different cohorts allows us to observe whether the prevalence of a particular condition is higher in one setting in contrast to another, such as metropolitan versus regional areas. Understanding the sources of these differences is often of central interest. Motivated by data from HealthNuts and the Barwon Infant Study, this paper develops methodology to enable the study of factors contributing to differences in disease prevalence across diverse settings and populations. Read more here.
LifeCourse Data and Access
Did you know?
LifeCourse cohorts hold over 500 million data points (excluding bioanalyses) spanning pre-conception to adulthood.
We provide several avenues for exploring the longitudinal cohorts at the Melbourne Children’s Campus.
Find out what data are available
Our metadata catalogue provides several different ways of exploring the available data and provides a crucial first step into understanding the available data across studies. Measures collected are organised into broad domains and aligned to relevant LifeCourse constructs, making it easy to browse and compare data between cohorts. Of course, it does not take the place of detailed study documentation, which should always be consulted when undertaking analyses.
Not sure what you’re after?
Explore our Cohorts to gain a bird’s eye view of our studies:
- An interactive timeline shows how long each cohort has been active
- Want to know more about a particular cohort? Delve in deeper to explore each cohort’s design and data collections
Interested in exploring the measures?
Our Measurement Library provides another opportunity to explore and identify measures used within each study, as well as areas of measurement consensus and convention across the cohorts.
Measures can be filtered by measure name, domain, construct and cohort. Entries can also be sorted by most frequently used within the LifeCourse cohorts.
Already know what you are after?
Use our Advanced Search Function to specify the data element(s) of interest across features such as what is being assessed, age of the participant, or source of the data.
Apply for data access
Found what you were looking for? Visit the Data Access page and lodge a request to start exploring a collaborative opportunity.
Specific questions about data or samples (e.g. quality or availability) can also be included in your enquiry, to assist in further refinement of your research question's design.
Data access requests are reviewed in accordance with each individual cohorts’ governance and ethics protocols, always holding the security of participant data to the highest standards.
Are you a cohort custodian?
Find more information on collating and updating your cohort's metadata by visiting the LifeCourse Cohort Metadata Hub.
Cohort custodians are also encouraged to explore the LifeCourse Data Access Hub for resources that can help to promote the appropriate reuse of your cohort's valuable data.
Data Management and Analysis
Did you know?
There are a wealth of experts and resources on campus to assist you with every stage of your data analysis journey, from planning to publishing.
MCRI’s Data Office and the Clinical Epidemiology and Biostatistics Unit (CEBU) can support researchers in planning for, managing and analysing their data.
Data management FAQs
Are there any guides or protocols I need to follow when developing my data management processes?
For an initial overview of research data management principles and practices, MCRI offer an Introduction to Research Data Management course available on Nucleus.
Visit DataConnect to access the appropriate tools and resources to support you in your data journey, and see how the Data Office can help you.
Two key resources to follow when developing your data management processes are:
- MCRI’s Data Governance Framework (DASUD) and,
- the Data Management Plan (DMP).
Find further information on MCRI’s policies on research conduct, and on the management of research data and materials under Ethics and Governance.
LifeCourse have also developed several resources to help cohort researchers plan, design and share their research, including:
- a Data Dictionary Resource to help cohorts design best-practice data dictionaries, and;
- a Data Sharing Planning Resource to help plan for future data sharing.
What does best practice in cohort data management look like?
Security and reproducibility are primary considerations to ensure best practice in data management.
- Security refers to protecting your data against loss and unauthorised access, in particular protecting the privacy and confidentiality of participant data in accordance with legislative requirements and best-practice guidelines.
- Reproducibility refers to maintaining data integrity and ensuring that data are handled and processed in a manner that is transparent and repeatable.
MCRI’s Data Governance Framework (DASUD) will help guide you through best practice cohort data management.
Also familiarise yourself with the requirements of the NHMRC and other relevant codes, and ensure you are adhering to all institutional policies.
I’m setting up a brand new cohort, what resources are available to support the development of my data management framework?
DataConnect can link you to a host of resources to support you in aligning to best practice in data management, including the Data Management Plan (DMP).
Resources are also available to provide useful guidance in designing best-practice cohort data dictionaries (Data Dictionary Resource) and planning for future data sharing (Data Sharing Guide).
A consultation with the CEBU team is a great place to start to discuss future analysis plans, which should inform your data management plans so that it is fit for future purpose. Additionally, register for one of their relevant short courses, particularly courses on Stata, R, and REDCap (see “Software FAQs” below).
My cohort was established some years ago, how can I update my data management system to contemporary standards?
Important considerations when updating your data management systems:
- Ensure that you meet all the data security and privacy requirements. Learn more at DataConnect.
- Ensure data handling workflows are updated, transparent and reproducible.
- Use the Data Management Plan (DMP) to revitalise your practices and align your project to the Data Governance Framework. Contact the Data Office if you’re unsure how to get started.
- Documenting your project (including its processes and data).
- The Data Dictionary Resourceis a good starting point for LifeCourse cohorts reviewing their data documentation, including suggested metadata fields and why they are useful to collect.
Reviewing your data sharing processes as well? Funders and journals increasingly expect that data will be shared (when appropriate). LifeCourse’s Data Sharing Planning Resource is a good first step when developing or reviewing your team’s data sharing plan, including tips on where to find further information.
Want more operational assistance? DataConnect’s Data Sharing page has MCRI’s data sharing procedure, which guides you through how to share data safely and securely.
To discuss further, contact the Data Office team.
My cohort is ready to be archived, what do I do?
Once your cohort data are coherently organised and thoroughly documented, collate the critical documents that describe the study and its operation (approved protocols, ethics approvals, SOPs, etc.) along with cleaned datasets and corresponding codebooks.
You may wish to maintain the data in a secure but accessible location within MCRI’s network storage, but also consider archiving into figshare (which can be done privately – see “Software” below) or other data repository platforms.
Contact the Archiving Service Desk for more information about the storage of hard copies of critical documents, such as consent forms. The Data Office are currently developing archiving procedures, which will be available soon.
Are there any central locations where I can store large datasets?
If your study has performed a bioanalysis (e.g. an omic analysis like genome wide SNP genotyping) that produces a large dataset, a central, restricted access, storage location can be arranged for your data. Contact MCRI IT for further information.
Data analysis FAQs
How can I obtain statistical input on my cohort analysis?
Getting high-level statistical input on your project from its inception to publication is critical in cohort research. This includes:
- Clarification and refinement of the research question;
- Development of the analysis plan, including epidemiological design principles and selection of the most appropriate methods;
- Reporting and interpretation of analyses.
It is also possible to gain hands-on statistical support to conduct analyses.
Haven’t worked with CEBU yet? Get in contact to organise an initial consultation to discuss your research question and potential requirements.
How can I grow my own statistical understanding in longitudinal analyses?
CEBU regularly runs a sequence of three core research methods short courses focused on basic statistical and epidemiological concepts that are critical to cohort research:
- Designing your research study
- Introduction to Biostatistics
- Observational Studies: Modern Concepts and Analytic Methods
Register for courses or read more via the MCRI website. A wealth of data management and statistics resources can be accessed via the CEBU resources website.
For training in more advanced analytic methods, check out course offerings from the Victorian Centre for Biostatistics (ViCBiostat), of which CEBU is a lead partner.
Where do I begin in planning my cohort analysis?
CEBU has developed an analysis plan template for the design of statistical analyses in observational cohort studies, particularly in life course epidemiology.
This is a useful tool in developing a robust analysis plan for your study.
How do I ensure my analysis is fully traceable and reproducible?
Making sure data analyses are reproducible ensures transparency of the research process and enhances confidence in research findings.
CEBU’s short courses on statistical software (in REDCap, Stata and R) provide a foundation in reproducible analyses, optimisation of analysis workflow via do-file/script management and file management for version control.
Software FAQs
What statistical software is supported on campus?
The two main statistical software packages supported by CEBU are Stata and R. These are the supported tools for data manipulation and analysis.
- Ris a free, open-source software environment,
- Stata can be accessed by researchers based on campus through the MCRI’s network licence. Find out how to obtain your copy via the MCRI’s Data Management Resources website.
More information about both these packages can be found on the intranet.
CEBU offers a number of short courses on both Stata and R – see the MCRI website for details.
Also check out CEBU’s Stata Tips page and graphing resources.
What data management software is available on campus?
The Data Office and CEBU support a number of data management software packages.
- For all data apps and tools available at MCRI visit DataConnect’s apps and tools site.
More information about CEBU’s data management resources and support can be found on the intranet.
Can I just use Microsoft Excel to store my data?
Never use Excel for data collection/storage or for manipulating data. Excel is a poor choice in relation to:
- Data integrity: Data easily moved between records with no audit trail of data manipulation steps.
- Consistency: Data type enforcement and validation checks are difficult to implement.
- Security: Weak password security and no permission controls.
- Usability: No metadata means no direct export to your statistical package.
Data Linkage
Did you know?
MCRI has a data linkage interest group on campus, open to all those wanting to explore issues and processes in data linkage.
Linkage to administrative data is increasingly being recognised as a powerful value-add to longitudinal cohort studies.
Where participants have provided consent, linking cohort data to available administrative datasets can enhance the range of information available to researchers.
Data linkage FAQs
How do I get started with data linkage?
There are a number of organisations that provide information on what data linkage is, how it can benefit your research, and how to get started:
- Australian Institute of Family Studies (AIFS)
- Australian Institute of Health and Welfare (AIHW)
- Centre for Health Record Linkage (CHeReL)
- Centre for Victorian Data Linkages (CVDL)
- Population Health Research Network (PHRN)
With feedback from researchers on campus, LifeCourse have also developed a number of guides outlining requirements and processes for linkage to various administrative datasets. These are frequently updated, so please contact the LifeCourse team for the latest versions.
How can I connect with others’ working in the linkage space?
LifeCourse hosts a Data Linkage Interest Group, where researchers from across the campus meet to connect and discuss various aspects of data linkage. Join us on the fourth Monday of the month, for presentations touching on a wide array of linkage issues and opportunities. Please contact LifeCourse to be added the mailing list.
I am interested in enriching my cohort data through linkage to administrative datasets. What are the ethical considerations?
Linking cohort data to an administrative dataset can add valuable context and meaning to your research. There are a number of ethical guidelines to first take into consideration. To discuss further, please contact the RCH REG team.
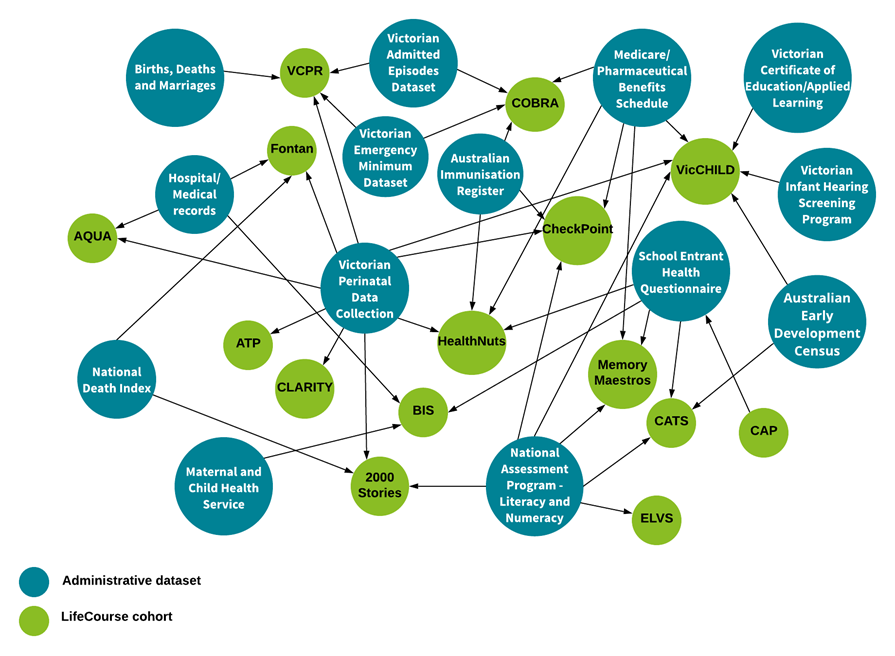
What are some of the datasets that other cohorts on campus linking to?
The figure below depicts a number of commonly used administrative datasets which have been successfully linked across LifeCourse cohorts. Click to zoom.
*This diagram is not exhaustive and reflects linkages as of 2017.
Biosamples
Did you know?
Over 80% of LifeCourse studies have collected biosamples as part of their study design.
MCRI has a number of resources to help cohort custodians collect, manage, store and analyse biosamples.
Participant consent will determine what biosamples can be collected, how long they can be stored, and what analyses can be performed. Refer to a study’s approved protocols, and participant information and consent forms. For additional assistance, see Ethics and Governance for contacts and resources or contact the MCBC Biobank Team
Biosample FAQs
My cohort is collecting biosamples for the first time, where do I start?
The Murdoch Children’s Research Institute Biobanking Facility (also known as the Melbourne Children's Bioresource Centre (MCBC) Biobank) supports Melbourne Children’s Campus research involving biosamples by providing advice and support through study planning, grant application justification and costing, and ethics approval.
The MCBC Biobank follows international best practice, in collaboration with the Biospecimens Advisory Committee (BAC), to perform:
- Sample processing
- Sample storage and management (via OpenSpecimen)
- -30°C, -80°C and liquid nitrogen storage
- Sample retrieval
Visit the Biobanking page for more information about the services offered through the MCBC Biobank, or contact the team directly at biobanking@mcri.edu.au. Consult with the MCBC Biobank Team as early as possible for guidance in planning your study, including how to future-proof your collection.
Is my cohort's biosample collection considered a biobank, and if so, is there anything I have to do?
If the collection of participant biosamples is covered by an extended or unspecified consent as part of a research study, this collection is considered a biobank and is covered by the Melbourne Children’s Biobank Governance Framework. The purpose of the framework is to aid researchers and clinicians in the establishment, management, oversight, and compliance of their biobanks. Visit the Biobanking contact us page to find out more.
Also, refer to the Biobank Advisory Committee Return of Incidental Findings Framework when preparing your biobank’s ethically defensible plan for return of findings.
My cohort collected biosamples some years back through a different facility, how do I update our storage and management protocols?
For the storage of biosamples, MCRI provides -80°C freezers as part of its infrastructure. The MCBC Biobank administrates the allocation of space to groups, in conjunction with the BAC. If you require additional -80°C freezer space, visit the request policy on the intranet. Appropriate sample tracking (as in, the collection and management of information about the samples, including storage location) is a requirement of freezer space allocation.
MCRI uses OpenSpecimen to manage sample information and storage on campus. OpenSpecimen replaced the previous system (FreezerPro) in late 2019.
- All information stored in FreezerPro was migrated to OpenSpecimen as part of the changeover
- If samples have been tracked through other mechanisms, contact the MCBC Biobank Team to discuss how you can start using OpenSpecimen
Setup and training sessions with a member of the MCBC Biobank Team is available to support cohorts looking to migrate to OpenSpecimen. Get in touch via biobanking@mcri.edu.au.
Biosamples in my cohort are now approaching the planned destruction date, what do I do?
Participant consent usually involves a specified length of time that biosamples can be stored for. Refer to the cohort’s approved study protocols, and participant information and consent forms. For further guidance, contact the Ethics and Governance team.
Contact biobanking@mcri.edu.au for further information on destruction procedures.
I’m thinking of performing a large-scale bioanalysis in my study, what do I do?
Examples of large-scale bioanalyses include genome-wide SNP genotyping, sequencing, expression analysis (e.g. RNA-Seq) or epigenotyping, proteomics, microbiomics, metabolomics, and metagenomics.
If cohort custodians are new to this type of analysis, and are unsure how to proceed, talking to campus experts is a good place to start. MCRI’s Scientific Services or the LifeCourse team can help in connecting you to relevant experts.
If you’re interested in conducting microbiome research, you can learn more about what’s happening on campus at Microbiome@MCRI on the intranet.
What do I need to consider before sharing my cohort’s biosamples with a collaborator?
Visit Research Design and Translation to find out more about what’s involved in establishing a new research collaboration. If you run a biobank, or your biosamples are considered part of a biobank (see above), please also refer to the Biobank Advisory Committee Return of Incidental Findings Framework when developing any sharing agreements.
Research Design and Translation
Are you curious?
Researchers at MCRI are finding new ways to collect data and disseminate research.
Ensure your research is reaching the right audience. There is support on campus to translate your research in more creative and innovative ways.
Research design FAQs
What measures are available for use in my cohort?
MCRI has site-wide licenses for a number of commonly used measures, including the:
- PedsQL
- SDQ
Licensing requires validated versions of the survey to be used. A number of these have been pre-programmed into REDCap for easy integration into data collection surveys. For any commonly-used measures not listed, contact the Data Office to discuss.
LifeCourse and the Data Office have also collaborated to draw together a standard set of demographic items, programmed into REDCap, that studies can consider for inclusion in their project. This common approach to gathering demographic information from participants aims to reduce the need for cohorts to ‘reinvent the wheel’ and creates opportunities for aligning and harmonising data across studies. Find out information here.
I want to purchase a measure for use in my cohort study. Are there any guidelines for doing this?
Contact the Data Office to ensure that the measure isn’t already covered by a site-wide license. If you still need to go ahead and purchase a measure, the Data office will work with you to ensure all requirements are met. Browse available measures here.
My study is establishing a new research collaboration. Where can I get help with setting up legal agreements?
Whether you are in need of a material transfer agreement, confidentiality agreement, research collaborative agreement, services agreement, contractor agreement or bespoke agreement, the Legal team have got you covered.
Visit the Legal page to find helpful templates for a range of agreements or email the Legal team if you wish to set up an agreement, have received an agreement that requires review or require any other legal advice. MCRI agreements should be reviewed and signed off by the Legal team before being shared externally.
My cohort study is implementing new design approaches. How can we make sure they align to the appropriate codes of conduct?
Issues and policies surrounding research integrity, conflicts of interest and professional conduct are outlined in MCRI’s Code of Conduct.
MCRI adheres to the Australian Code for the Responsible Conduct of Research, as issued by the National Health and Medical Research Council.
Additional policies and frameworks are in place for ethical breaches and queries regarding misconduct. Find out more in the Framework for Handling and Resolving Breaches of the NHMRC Code and Scientific Misconduct at the Royal Children’s Hospital Campus, or if you’re looking for specific advice email the Legal or Risk teams.
How can I connect with other cohort managers and coordinators?
LifeCourse hosts a Cohort Coordinators Forum, where cohort coordinators and project managers meet to share knowledge and expertise, and troubleshoot specific design issues arising in the cohorts context. For more information about the LifeCourse forums, visit the Forums and Training tab.
Contact LifeCourse to be added to the mailing list.
Research Translation FAQs
How do I integrate planning for impact into my research design?
Learn more about translation and impact via the campus Research Hub. Here you will find templates and steps to help you understand the importance of planning for impact, mapping out your research impact, developing a program logic, and creating a feasible and comprehensive plan for research impact.
You may also consider joining the Melbourne Children’s Knowledge Translation and Impact network, which is a campus community group who share tools, tips and examples of good practice in this area.
The can help you learn more about development plans, tech transfer, intellectual property management, commercialisation and/or how to engage with investment and industry partners. To get in contact email Innovation@mcri.edu.au.
Our team has a unique idea for the translation of our research. Who can assist us in bringing this idea to life?
Get in contact with the Innovation team to discuss all of your ideas! You can fill out a new idea form to get the ball rolling.
The Innovation team is working with a vast network of advisors, champions and mentors and with them they provide expertise in:
- Innovation opportunity assessment
- Intellectual property licensing and contracting
- Product management
- Funding generation
- Partnership creation & management
- Traditional deal licensing
- Sponsored research collaboration
- Digital enablement
The Legal team can assist with agreements and legal advice related to these areas.
You can also stay up to date with all the latest MCRI Innovation news by signing up to their mailing list.
Planning and protecting your intellectual property before you publish any of your findings is also a crucial step. To gain an understanding of this process, contact the Innovation team, or contact the Head of Intellectual Property, Jacquie Crawford.
How do I maximise the real world impact of my research findings?
The Innovation team’s mission is to empower and connect people at the Melbourne Children’s campus to help them develop and translate their research ideas into real-world applications that benefit children and their communities.
They have experience in facilitating real-world applications like:
- Therapeutics
- Diagnostics
- Devices
- Digital health
- Policy and program development
- New models of care
- Clinical guidelines and procedures
- Social and community enterprises
If you are looking for Innovation funding and training opportunities, click here or contact the Innovation Team.
Ethics and Governance
Did you know?
All LifeCourse cohorts are required to maintain the highest standards of ethical conduct.
On-campus resources are available to assist you in navigating ethics and governance approvals for new or existing cohort studies.
Research Ethics and Governance
The Royal Children’s Hospital Research Ethics and Governance (REG) has templates and guides to support your next ethics application or amendment. This includes guidance on informed consent and how to write in plain language. Ethics approval for each of the LifeCourse cohorts will primarily go through the Royal Children's Hospital Human Research Ethics Committee (RCH HREC).
The Clinical Research Development Office (CRDO) also provides education and training on regulatory frameworks for research.
Ethics and governance FAQs
Do I need ethics approval?
Are you launching a cohort study, planning to collect additional data from your cohort, or acquiring additional data through linkage? If the answer is yes, you need ethics approval. The REG website can help you determine which type of application you need to complete.
But I’m only analysing cohort data, do I really need ethics approval?
Even if you’re simply analysing cohort data rather than collecting your own data, it is more than likely you’ll need ethics approval.
If you work outside the cohort’s study team you’ll need to consider whether your intentions for the data extends beyond the study’s original aims. If so, you may need to submit a new ethics project covering your analysis of the data. Speak to the cohort custodians to discuss your next steps.
Depending on the nature of your research, it may qualify as a low or negligible risk application. Be sure to check out the great resources available through RCH Ethics and Governance and CRDO.
As an example, this ethics protocol outlines the proposed analysis of secondary cohort data for the LifeCourse-COVID-Wellbeing research program. This protocol outlines who will view the data as part of the secondary data analysis, what research question the data will be used to answer, and ethical issues relating to data management and security.
Submitting an ethics application for a proposed analysis is usually the responsibility of the data user. Some cohorts require a copy of the approval letter and protocol before transferring data to make sure this approval is in place.
I want to collect additional waves of data for my cohort study. Do I need to add to or change the existing protocol?
With long-running studies, ethics approvals generally need to be amended over time as new waves of data collection are planned. For example, you may need to change your study aims or objectives or add a new data collection method. The REG website tells you how to submit an amendment.
Sometimes, your change may be significant enough that a new application will need to be considered. This can happen even for a long-running study. For example, this might occur if the cohort is being repurposed to address a new research question.
If you are unsure about whether a change requires an amendment or new application, or have any further questions, please contact the RCH REG team.
What does informed consent mean for participants in long-running cohort studies?
Because participants remain engaged with cohort studies over long periods of time, you need to negotiate informed consent in an ongoing way.
This includes, for example, updating participants about changes to how their data will be collected, stored, and utilised.
Can I use social media to trace participants who have been lost to my cohort?
Losing contact with participants over time, such as when families move to a new house or change their phone number, is a major concern for long-running cohort studies.
Social media is emerging as one potentially helpful technology available to assist in contact tracing. The RCH has guidelines about ethical use of social media in research. Before using social media for your research, contact the MCRI or RCH Communications team to access their policies and guidelines on social media use.
I am interested in enriching my cohort data through linkage to administrative datasets. What are the ethical considerations?
Linking cohort data to an administrative dataset can add valuable context and meaning to your research. There are a number of ethical guidelines to first take into consideration. To discuss further, please contact the RCH REG team.
How do I complete and submit my ethics form?
You must complete and submit your ethics application through the Ethics Review Manager (ERM). See the ERM user guide for more information.
Is there a deadline for my ethics application?
New ‘greater than low risk’ applications are reviewed on the first Monday of each month. Other applications must be submitted by the relevant dates. The REG website also has a list of these dates.
There are no deadlines for new quality assurance (clinical audits) and low and negligible risk applications. The RCH REG office are happy to receive these at any time.
Funding Opportunities
Did you know?
There may be more funding options available for your project than you’re currently aware of!
MCRI’s Grants Office can assist in finding funding opportunities, providing advice on applications, and ensuring administrative and legal requirements are followed.
Funding opportunities FAQs
Where can I find out about potential funding opportunities?
Use the MCRI Grants Calendar and Research Professional to seek out funding opportunities for your cohort research, and come and visit the MCRI Grants Office. Internal funding opportunities are also available.
Where do I start in preparing my application?
The MCRI Grants Office can assist with reviewing the application and ensuring it complies with the funding opportunity guidelines.
Contact the Grants Office as early as possible to discuss your application and follow the process for application submissions. You will need to provide an Internal Approval form signed by your Theme Director and draft for review.
The Grants Office intranet pages are a great resource for planning and writing grant applications including budgeting templates and descriptions of the MCRI research environment.
How do I consider the potential research impact of my project?
Have you determined what the impacts and outputs of your research will be, beyond publications? Have you considered how you will influence science, policy and practice through your research?
If not, get in touch with the Melbourne Children’s Knowledge Translation and Research Impact Project for guidance in outlining and demonstrating your potential research impact to funders.
My funding application was successful – what do I do now?
Congratulations! Let the Grants Office know when you are successful with a funding application. They will assist you with the formal documentation required for the post-award steps your project.
My funding application was not successful – what do I do now?
Take a day to process the rejection of your application. In this competitive environment, funding bodies receive many, more applications than they can fund. In recent major NHMRC funding rounds, over 80% of applications were deemed fundable, however only 11% were successful.
Carefully read any feedback provided and re-read your application – you may uncover weak spots that were not obvious under the pressure of a grant deadline.
And, try again. Develop your ideas, match your project to other funding opportunities or reapply to the same opportunity in the next round; but always try again. Researchers often find that ‘it never rains, but pours’, where one grant success snowballs into further funding success.
Ready to get in touch with the Grants Office?
The Grants Office can be contacted any time, get in touch to discover what opportunities you might be eligible for!
Open Science
Did you know?
Globally, research paradigms are shifting to adopt Open Science principles.
LifeCourse is taking steps to support an Open Science culture.
Open Science Practices
Open Science is a growing movement which aims to increase the transparency of research processes to advance scientific discovery. LifeCourse is making efforts to move in this direction, while ensuring that best practice ethical and data security standards are maintained at all times.
Open Science includes a number of key principals which are increasingly being adopted throughout LifeCourse working practices. Keep reading to learn more.
What is pre-registration?
A preregistered design includes details of the research design, research questions, and analytic approach. Preregistering does not prevent researchers from shifting course and adapting to new information. Rather, it is a foundation for recording how and why the plan changed.
The CEBU-developed analysis plan template provides a framework for stepping through the aims, key variables, and design of an analysis, before it is undertaken.
What can we gain from open materials?
Open materials is about making publicly available the components of the research methodology, like what data were collected and how, and how that data was analysed. This makes study findings more transparent and reproducible.
Which measures are used by our cohorts and when? Our cohorts have made this information visible and browsable. Visit the Measurement Library, or browse by cohort.
We are also helping data custodians design their processes more consistently and fully describe their data. Our Data dictionary resource helps custodians to set up their data dictionaries in adherence with best-practice standards, minimising the duplication of data management work in the future.
What do we mean by open data?
Balancing the value of transparency with the need to protect participant data is critical to sharing data responsibly.
LifeCourse has a clear and transparent procedure for researchers to apply for access to the data within our cohorts, that also ensures all safeguards for participants are maintained.
Our guide on Planning for data sharing is helping data custodians to embed and future-proof a plan for data sharing early.
How can we achieve open access to research outputs?
Providing free access to papers and other research outputs online removes cost and access barriers. For example, an increasing number of journals allow for the publication of pre-prints.
LifeCourse researchers have access to figshare, an online repository enabling researchers to archive or publish research outputs in a discoverable, shareable and citable manner.
Meet the Team
Did you know?
LifeCourse started in 2013, collaborating across cohort investigators, project managers and data users.
LifeCourse is managed by a group of researchers and professionals based at MCRI.
LifeCourse Team
A small and efficient team work to maintain and optimise the LifeCourse infrastructure, as well as undertake research that demonstrates the power of the platform to generate new insights into health pathways over the life span.

Dr Meredith O'Connor - Senior Research Fellow and Program Lead
Meredith's research program draws on LifeCourse cohorts to understand influences on mental health, with a particular focus on how cohorts can be analysed in tandem to gain new insights. Meredith also oversees the LifeCourse data infrastructure, developing systems that enable reuse of these valuable longitudinal data assets to facilitate and foster real world impact. In addition, she contributes to the strategy and design of prominent longitudinal studies in Australia, including serving as a Scientific Advisor for the Longitudinal Study of Australian Children since 2018.

Tehani Paiva - LifeCourse Initiative Coordinator
Tehani joined the LifeCourse team in January 2019. She currently manages key LifeCourse projects that develop data infrastructure and resources supporting LifeCourse researchers. She has specialised knowledge in the measures and instruments used by cohorts, and manages the cohort metadata catalogue where researchers can browse this information. She also works with cohort custodians to foster the sharing of knowledge and approaches across teams, and assists in the curation of the LifeCourse forums program.

Ella Woodbridge - Project Assistant
Ella joined the LifeCourse team as a Project Assistant in 2024. In this role, she liaises with cohort custodians to update their metadata, manages and promotes LifeCourse forums, and maintains LifeCourse website resources. She has a background in education and disability support. She is undertaking a Master of Public Health and is interested in health intelligence and reproductive health.
LifeCourse Scientific Leads
The LifeCourse team is supported by leading researchers who provide expertise across diverse research disciplines. They play a pivotal role in supporting the LifeCourse initiative by identifying innovative research directions, particularly participation in international cross-cohort consortia; advocating for the platform; ensuring alignment with other Campus efforts; and fostering relationships with key stakeholders.

Professor Craig Olsson (Founding Investigator) - Mental health
Craig led the early development of LifeCourse between 2014-2018 as founding investigator of the LifeCourse platform. He holds an NHMRC Investigator Grant and leads The Australian Temperament Project Generation 3 Study (est. 1983), one of Australia’s longest running studies examining the developmental origins of mental health and disorder. He also leads the Intergenerational Cohort Consortium of multigenerational cohort studies, and is National Convenor of the Australian Research Alliance for Children and Youth (ARACY) Longitudinal Studies Network.

Professor David Burgner (Founding Investigator) - Infection, inflammation and chronic disease
Dave is a paediatric infectious diseases clinician scientist. He holds an NHMRC Investigator Grant and is one of the lead researchers on the Barwon Infant Study, Child Health Checkpoint and a number of COVID-19 related projects. He also co-leads a number of national and international cross-cohort studies. His research focus is on understanding early life determinants of infection and inflammation, and their role in the development of conditions like heart disease and obesity.

A/Professor Margarita Moreno-Betancur - Research methodology
Margarita is co-Director of the Clinical Epidemiology and Biostatistics Unit (CEBU). She leads an integrated program of methodological and collaborative research, currently supported by an NHMRC Investigator Grant (2022-26). With the goal of bringing methodological excellence to observational research on campus, she has developed a hub of innovation and expertise in statistical areas that are key for these studies, such as causal inference.

Professor Andrew Sinclair - Data infrastructure
Andrew is Deputy Director of the Murdoch Children's Research Institute (MCRI) and a Professor in the Department of Paediatrics at The University of Melbourne. In these roles and as Executive Director of the Victorian Clinical Genetics Service at the Royal Children's Hospital, Melbourne, he is using genomic information to improve healthcare for children and their families. He also leads MCRI’s efforts to support best-practice data management and governance approaches.
Other critical contributors
LifeCourse is a collaboration between the Murdoch Children’s Research Institute (MCRI), the University of Melbourne, and the Royal Children's Hospital, located at MCRI. We acknowledge all collaborators who have contributed to LifeCourse, especially cohort data custodians and their participants, and our LifeCourse Initiative funders, the Royal Children's Hospital Foundation and MCRI. LifeCourse is a collaborative effort, and we would like to thank everyone for their ongoing engagement and support.
Special thanks to these previous and ongoing contributors to LifeCourse:
Executive champions
Sharon GoldfeldAndrew Sinclair
Past leadership
Katie AllenLeanne Mills
Melissa Wake
Advisory group members
Andrew GrimesAnne-Louise Ponsonby
Ben Ong
Jatender Mohal
Jennifer Piscionere
John Carlin
Justine Ellis
Kate Lucas
Luke Stevens
Michael Poidinger
Nitya Phillipson
Revital Rosenberg
Richard Saffery
Shyamali Dharmage
Steven McConchie
Research contributors
Harindra JayasekaraKatherine Lange
Marnie Downes
Sarah Arnup
Infrastructure contributors
Alana DeeryAngela Pezic
Anna Booth
Anna Duncan
Emma Jarosz
Gabriella Tikellis
Hannah Mcglashan
Joanne Ryan
Keri Little
Kerry Haynes
Shirani Sivarajah
Will Siero
Web development
Adam LeadouxDhanuka Kaluarachchi
Hilianty Tardjono
Lucy Anderson
Vijay Mudapilaval Rajendranath